6 Types of Electronic Source Data
This article describes 6 types of electronic source data originators in clinical trials. It also discuses what information should be attributed to data elements from electronic sources and ends with a view of where the industry might go next.
Contents
The clinical trials industry has collected data electronically for many decades but this process was heavily dependent on a form being printed so handwritten results could be recorded and manually entered into an EDC (Electronic Data Capture) system.
The industry is moving towards automation with the increase in systems designed for sites to enter data directly at the point of care, known as Electronic Source Data, or eSource for short. We are also seeing a rise in systems connected to Electronic Health Records (EHR) which are used to retrieve patient medical details. Lab results have been imported for many years from Laboratory Information Management Systems (LIMS); and more recently the rise of decentralised trials has seen more patients entering their own data, known as Patient-Reported Outcomes (PRO).
Clarifying the difference between EDC and eSource
In a nutshell, EDC tends to be controlled by the sponsor or Contract Research Organisation (CRO) whereas eSource is a replacement for paper and tends to be controlled by the site. Where things get confused is the middle ground where the CRO owns sites that are collecting data, but generally this is where the separation of ownership sits.
Most EDC systems are not designed for site workflows because they lack the ability to separate subject identifiers from Protected Health Information (PHI) which are used to contact the volunteer. Another reason why EDC systems tend not to be a good fit for site processes is the inability to schedule visits within a site calendar and generate reminders for the volunteer to attend appointments. Real-time data collection can be harder in an EDC system because access to data entry screens tends to be by form rather than by visit. The final limiting factor of EDC systems for site data collection is that sites are not permitted permanent control over the source data.
Examples of source data originators
In an effort to modernise clinical investigations, the FDA produced guidance in 2013 titled Electronic Source Data in Clinical Investigations. The guidance provides the following examples of source data originators:
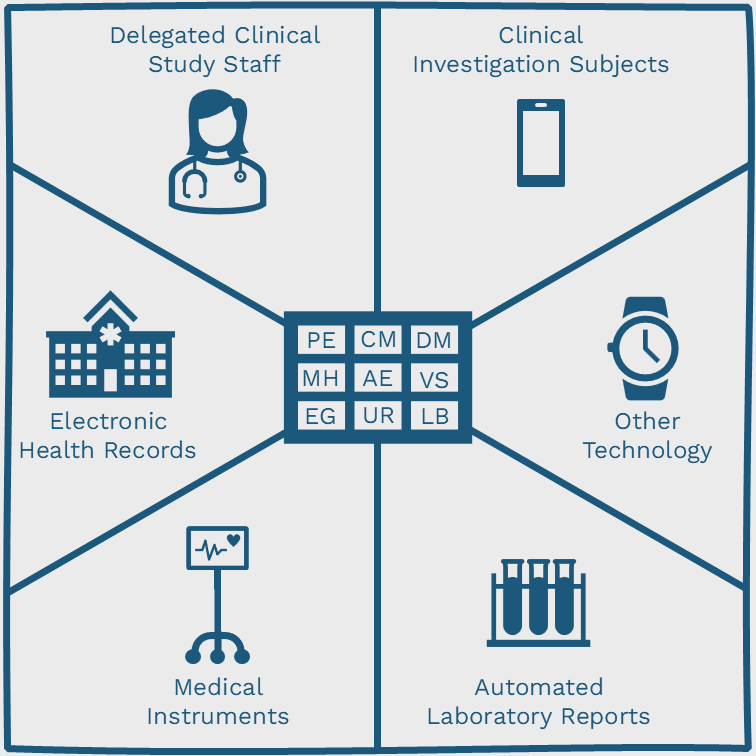
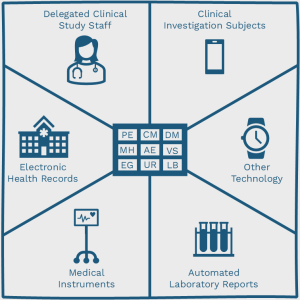
- Delegated clinical study staff
- Clinical investigation subjects (PRO)
- Medical instruments
- Electronic health records (EHR)
- Automated laboratory reports (LIMS)
- Other technology (e.g. wearables)
The following image graphically shows the different types of data originators, revolving around various SDTM domain codes.
Data can be entered into the eCRF (electronic Case Report Form) either manually or electronically. The following table provides a handy reference to understand what the data originator is, and what is defined as the source, according to the FDA guidance.
| Method of Data Entry into the eCRF | Originator | Source |
| Direct Entry of Data | Clinical study staff | eCRF |
| Automatic Transmission of Data from Medical Instruments | Medical instrument | eCRF |
| Transcription of Data from Paper or Electronic Sources | Person transcribing data | Electronic or paper documents |
| Direct Transmission of Data from the EHR | EHR | EHR |
| Transmission of Data from PRO Instruments | Subject | eCRF |
Identifying where the data came from
A data element in an eCRF represents the smallest unit of observation captured for a subject in a clinical investigation. For data elements where the source is derived externally, the following minimum details must be included:
- Whether the data was manually or automatically entered
- Timestamp of when the data was entered
- Which subject the data belongs to
I would argue that in order to provide complete transparency, the type of originator should include a clear identification of the method such as the make and model of a medical instrument; and if a user can also be attributed to the external data then this would also be helpful.
Extracting electronic source data
The good news is that once electronic source data has been entered into an eCRF it is very easy to extract the data using tools included in the EDC system. If you don’t have the required tools then why not schedule a conversation with us to discuss your needs. If you want to learn more about extracting clinical data why not read this blog post.
Hang on, what about the protocol time?
In later phase trials the protocol dictates procedures should be collected on a certain day before or after dosing. For early phase trials the protocol will require procedures are collected at a certain time (known as the nominal or protocol time), either side of the dose.
When procedures must be collected at a specific time it is important for staff to be prompted to collect the data point within time tolerances specified either side of the protocol time.
This is not a major concern for later phase trials where procedures must only be entered on a certain day so this functionality is unlikely to be added to large EDC systems. There will however continue to be demand within early phase trials for this type of functionality so if you’re looking for advice in this area please schedule a conversation to learn more.
What’s next for electronic source data?
The ACDM recently conducted a poll among LinkedIn connections and asked “when was the last time you used paper CRFs in a clinical trial?”. Out of 273 respondents, only 11% are still using paper CRFs so this clearly shows the majority of trials are being entered directly into electronic systems (link to post with results). This is good news for the natural environment as we reduce our reliance on transcribing data from paper; but it doesn’t answer the question “what’s next for electronic source data?”.
Alongside the continued expansion of companies offering the ability to import medical data from EHRs, I believe we will see an increase in EDC platforms importing electronic source data from site eSource systems. This will allow EDC systems to focus on central functionality like monitoring and analysing data; and will provide more opportunities for eSource systems to provide site-specific functionality for running clinical trials more efficiently and effectively at the point of care.
Available eSource Systems
The following companies helped form my thoughts around this post so please visit them for more information. I am in no way compensated for including these companies in this list.
- Ignite Data https://ignitedata.com/
- Clinical Ink https://www.clinicalink.com/
- Realtime eClinical Solutions https://realtime-eclinical.com/
- Open Clinica https://www.openclinica.com/
- CRIO https://clinicalresearch.io/