
Category: Life Sciences
-
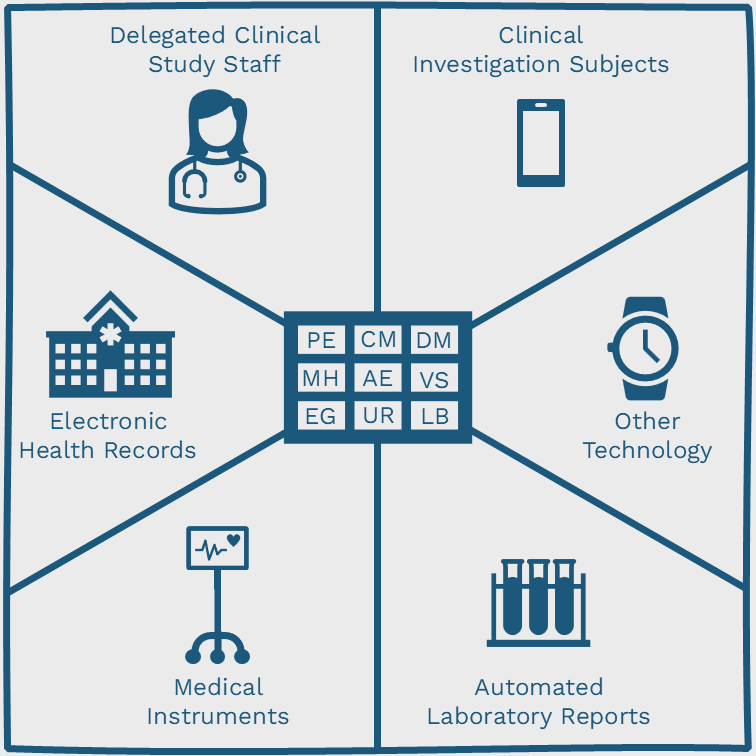
What is Electronic Source Data in Clinical Trials?
Read More: What is Electronic Source Data in Clinical Trials?6 Types of Electronic Source Data This article describes 6 types of electronic source data originators in clinical trials. It also discuses what information should be attributed to data elements from electronic sources and ends with a view of where the industry might go next. The clinical trials industry has collected data electronically for many…
-

What is the deal with Dataset-JSON?
Read More: What is the deal with Dataset-JSON?Unpacking the Complexities of Clinical Data Submission Standards There is a storm brewing in the world of CDM (Clinical Data Management) and regulatory submissions. Since I came across the news that the FDA are considering Dataset-JSON as a new standard for submission I have conducted a lot of research to distil the wealth of information…
-

How do I extract clinical data from old systems?
Read More: How do I extract clinical data from old systems?Efficient Extraction of Clinical Data from Old Systems This article explores the challenge keeping clinical data in an old system and suggests various methods of extraction. Challenges of Old Systems Managing data in an old system presents several challenges that impact the efficiency and effectiveness of managing a clinical trial. Some of the key issues…
-

What is Clinical Data Management (CDM)?
Read More: What is Clinical Data Management (CDM)?People, Processes, & Disciplines of Clinical Data Management Last week I had the pleasure of attending the ACDM conference in Copenhagen, Denmark. On my return to the UK I started to wonder if the term Clinical Data Management (CDM) is clearly understood. In my opinion it does not only refer to the people and processes…
Search
Popular Posts
-
What is Electronic Source Data in Clinical Trials?
6 Types of Electronic Source Data This article describes 6 types of electronic source data originators in clinical trials. It also discuses what information should be attributed to data elements from electronic sources and ends with a view of where the industry might go next. The clinical trials industry has collected data electronically for many…
-
Why do we need so much functionality in software?
So Much Functionality in Software This article explains how much of software functionality we tend to use and explores why there are so many features to choose from. We end with offering a few suggestions for how to expand your knowledge of unused features. Unused Functionality in Software Research conducted by The Standish Group about…
-
What is the deal with Dataset-JSON?
Unpacking the Complexities of Clinical Data Submission Standards There is a storm brewing in the world of CDM (Clinical Data Management) and regulatory submissions. Since I came across the news that the FDA are considering Dataset-JSON as a new standard for submission I have conducted a lot of research to distil the wealth of information…


